Background: The outcomes for patients (pts) with myeloid malignancies who relapse after allogeneic hematopoietic cell transplantation (HCT) is poor, and relapse occurs more frequently for those with high-risk mutations or cytogenetics. The oral selective BCL-2 inhibitor and BH3 mimetic venetoclax (VEN) increases mitochondrial apoptotic priming even in chemoresistant myeloblasts. Reasoning that VEN would selectively increase the anti-leukemic effect of HCT conditioning chemotherapy without undue toxicity, we evaluated the safety and efficacy of adding VEN to fludarabine and busulfan (FluBu2) reduced intensity conditioning (RIC) chemotherapy. Here, we report on the completed dose-escalation and expansion cohorts from the phase 1 trial.
Methods: Pts received escalating doses of VEN (dose level (DL)1, 200 mg on Days -8 to -3 (n=3); DL2, 200 mg on Days -8 to -2 (n=3); DL3, 400 mg on Days -8 to -2 (n=16)) in combination with FluBu2 (fludarabine 30 mg/m2/d on Days -5 to -2 and IV busulfan 0.8 mg/kg bid on Days -5 to -2), followed by PBSC infusion on Day 0 and tacrolimus/methotrexate GVHD prophylaxis. Eligible pts included those with adverse risk AML per ELN criteria or secondary AML in complete remission (CR); MDS (t-MDS; Int-2 or higher IPSS; presence of TP53 or RAS pathway gene mutation) or MDS/MPN (including +8; chr 7 abnl; complex karyotype; or ASXL1 mutation) with £10% blasts pre-transplant; and an 8/8 HLA-matched donor. Dose-limiting toxicity (DLT) was defined (first dose of VEN until Day +28) as any treatment-related death, failure to achieve an absolute neutrophil count (ANC) ≥ 500/mL on 2 consecutive occasions, or any gr 4 non-heme toxicity or tumor lysis syndrome. Targeted sequencing of 88 genes on a clinical assay (sensitivity 1-3%) was performed on pre-VEN and day +100 marrow samples. Flow cytometry-based BH3 profiling was performed on pre-VEN treatment bone marrow in pts with measurable residual myeloblasts.
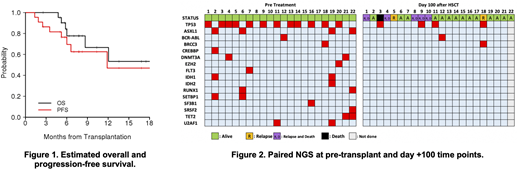
Results: Twenty-two pts (median age 64 y, range 25-71) were treated including 9 AML, 11 MDS, and 2 MDS/MPN cases. 45% of the cohort had an ECOG 2 (n=10). A mutation in TP53 was identified immediately pre-transplant in 55% of pts (n=12). Median HCT-CI score was 4 (range 1-8). 35% and 65.2% had intermediate- and adverse- cytogenetic risk. 20 received HLA-matched unrelated and 2 received HLA-matched related grafts. Median bone marrow leukemia blasts prior to study was 5% (range 5-10). The most common grade 3 or higher non-heme toxicity observed were grade 3 ALT (n=3), diarrhea (n=3), and rash (n=4). Neutrophil engraftment was achieved in all 22 patients (100%). Median days to neutrophil and platelet recovery were 15 (range 12-33) and 14 (range 10-19), respectively. Median donor-derived myeloid chimerism at day +28 was 100% (range, 97-100). No DLTs were observed at DL1-DL3. Acute and chronic GVHD were detected in 12 (GrI, n=7; GrII, n=4; GrIII, n=1) and 6 patients (NIH moderate, n=4; severe, n=2), respectively. At day +100, 16 out of 21 (76%) evaluable patients were in CR. Of the total 22 pts treated to date, 6 have died (5 from disease relapse and 1 from GVHD-related complications). Overall, 7 of 22 pts have relapsed; 2 of these 7 pts subsequently achieved CR2 (one after tapering immunosuppression alone and another after receiving chemotherapy). Median follow-up time among 16 surviving pts is 7.4mo (range, 2.6-19.5). Median OS has not been reached. Median PFS is 11.9 mo (95% CI 6.0 mo, not estimable) (Fig 1). For the entire cohort, the 6-month OS and PFS were 84% and 76%, respectively. Univariable analysis demonstrates pts with an ECOG 0/1 predicted OS and PFS in univariate analysis (p=0.025, 0.001, respectively). Of 21 pts with available paired pre-VEN and day +100 samples, 5 had persistent mutation by NGS (Fig 2). BH3 profiling analysis is underway.
Conclusions: The addition of VEN (400 mg; RP2D) to the backbone of FluBu2 conditioning chemotherapy for allo-HCT is not associated with increased toxicity. VEN/FluBu2 combination demonstrates promising clinical activity supporting further evaluation for high risk disease features. To further reduce disease relapse, we are testing VEN/FluBu2 conditioning chemotherapy with VEN/hypomethylating agent maintenance therapy.
Garcia:Eli Lily: Research Funding; Pfizer: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cutler:Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kadmon: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Medsenic: Consultancy, Membership on an entity's Board of Directors or advisory committees; Generon: Consultancy, Membership on an entity's Board of Directors or advisory committees; Mesoblast: Consultancy, Membership on an entity's Board of Directors or advisory committees. Stone:Astellas: Consultancy; Argenix: Other; Syros: Consultancy; Novartis: Consultancy, Research Funding; Janssen: Consultancy; Celgene: Consultancy, Other; Daiichi-Sankyo: Consultancy; Syndax: Consultancy, Research Funding; Macrogenics: Consultancy; Stemline: Consultancy; Pfizer: Consultancy; Trovagene: Consultancy; Aztra-Zeneca: Consultancy; Jazz: Consultancy; Actinium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Arog: Consultancy, Research Funding; Biolinerx: Consultancy; Takeda: Other: DSMB; Syntrix: Other: DSMB; Agios: Consultancy, Research Funding; Gemoab: Consultancy; Abbvie: Consultancy, Research Funding. Koreth:EMD Serono: Consultancy; Cugene: Membership on an entity's Board of Directors or advisory committees; Regeneron: Other: Research Support; Equillium: Consultancy; Biolojic Design Inc: Consultancy; Therakos: Membership on an entity's Board of Directors or advisory committees; BMS: Other: Research Support; Amgen: Consultancy; Moderna Therapeutics: Consultancy; Miltenyi: Other: Research Support; Clinigen: Other. Nikiforow:Nkarta Therapeutics: Honoraria; Novartis: Honoraria; Kite/Gilead: Honoraria. Steensma:Takeda: Consultancy; BMS/Celgene: Consultancy; Onconova: Consultancy; Arena: Current equity holder in publicly-traded company; CRISPR: Current equity holder in publicly-traded company; Aprea Therapeutics: Research Funding; Arrowhead Pharmaceuticals: Current equity holder in publicly-traded company; Astex Pharmaceuticals, Onconova Therapeutics, Pfizer, Stemline Therapeutics, Summer Road: Consultancy; H3 Biosciences: Research Funding. Letai:AbbVie: Consultancy; AstraZeneca: Consultancy; Chugai: Other; Dialectic: Membership on an entity's Board of Directors or advisory committees; Flash Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Zentalis: Membership on an entity's Board of Directors or advisory committees. Lindsley:Jazz Pharmaceuticals: Consultancy, Research Funding; MedImmune: Research Funding; Takeda Pharmaceuticals: Consultancy; Bluebird Bio: Consultancy. Soiffer:Celgene: Other; Juno: Other; Alexion: Consultancy; Novartis: Consultancy; VOR Biopharma: Consultancy; Mana Therapeutics: Consultancy; Precision Bioscience: Consultancy; Cugene: Consultancy; Rheos Therapeutics: Consultancy; Gilead: Consultancy; Be The Match/National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Kiadis: Membership on an entity's Board of Directors or advisory committees. DeAngelo:Forty-Seven: Consultancy; Shire: Consultancy; Blueprint Medicines Corporation: Consultancy, Research Funding; Takeda: Consultancy; Abbvie: Research Funding; Glycomimetics: Research Funding; Pfizer: Consultancy; Novartis: Consultancy, Research Funding; Incyte Corporation: Consultancy; Jazz: Consultancy; Agios: Consultancy; Amgen: Consultancy; Autolos: Consultancy.
venetoclax with conditioning chemotherapy in the context of a clinical trial
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract